Your cart is currently empty!
Tag: Bristol

SOP for Out of Specification (OOS)
SOP for Out of Specification (OOS) SOP for the procedure for handling, investigating, and reporting test results, which do not meet the specification. This SOP is applicable to all out of specification results during the analysis of raw materials, packaging material, finished products, stability study samples, and any unusual results observed for test parameters This…
Registration of the samples received by the QC physical-and-chemical and microbiology laboratories for testing
Registration of the samples received by the QC physical-and-chemical and microbiology laboratories for testing OBJECTIVE To provide the registration of samples received by the QC physical-and-chemical and microbiology laboratories for testing. SCOPE This SOP is applicable to the registration of samples received by the QC physical-and-chemical and microbiology laboratories for testing. RESPONSIBILITY Quality Control Analyst…
Physical-and-chemical test analysis analytical reports
Physical-and-chemical test analysis analytical reports OBJECTIVE To provide the procedure analytical reports/calculation sheet. SCOPE This SOP is applicable to the the procedure analytical reports/calculation sheet. RESPONSIBILITY Quality Control Analyst shall be responsible for following the procedure mentioned in this SOP. ACCOUNTABILITY QC Head and QA Head shall be accountable for compliance of this SOP. ATTACHMENTS …
ACCELERATED STABILITY AND SHELF-LIFE CALCULATION
ACCELERATED STABILITY AND SHELF-LIFE CALCULATION Objective: To lay down a method for accelerated stability and to find out the shelf life of the product. SCOPE: To ensure that the procedure method for accelerated stability and to find out the shelf life to the product in pharmaceutical Industries. RESPONSIBILITY: QA and QC personnel shall be responsible…
Procedure of the control samples keeping
The procedure of the control samples keeping To provide the procedure of adherence to the conditions and period of control samples keeping. This SOP is applicable to the procedure of adherence to the conditions and period of control samples keeping. ATTACHMENTS Control sample room entry & exit registration – Attachment-I Control sample: storage and…

The operating procedure of the air sampler in Micro department In Pharma
The operating procedure of the air sampler in Micro department In Pharma To lay down the operating procedure of the air sampler. This procedure is applicable for microbiological monitoring the air in the production zone with the help of the air sampler. PROCEDURE: General cleaning Clean the surface with a soft cloth, if required, use…

Record of pressure in the microbiological laboratory in Pharma
Record of pressure in the microbiological laboratory in Pharma To lay down the procedure of pressure record in the microbiological laboratory rooms. It is for monitoring the pressure difference between rooms of the lab. PROCEDURE: The pressure difference between the rooms is determined by the laboratory using a manometer Magnehelic. Before the account for the…

Operation of pass box transferring of materials in Microbial lab of Pharma Industry
Operation of pass box. transferring of materials in Microbial lab of Pharma Industry To lay down the pass-box operation procedure. This procedure is applicable when passing the material from one room to another using the pass-box. PROCEDURE: General cleaning After use, wipe the inside of the folded-box clean dry rag. After completing the mechanical cleaning…

Safety working in the microbiological laboratory In Pharma
Safety working in the microbiological laboratory In Pharma Providing a safe work environment to employees. The personnel of the microbiological laboratory. PROCEDURE: To work in the laboratory allowed a person for a recent preliminary medical examination, an introduction, and initial instruction in the workplace on occupational safety, special education, if necessary (including safety), and validation…

Requirements to personnel of the miсrobiological laboratory
Requirements for personnel of the miсrobiological laboratory Set requirements to personnel of the microbiological laboratory Determine the qualifications of personnel, their rights and responsibilities, and also official relationships. PROCEDURE Requirements, rights, and duties of employees of the microbiological laboratory are determined in duty regulations. On arrival at work, the employee must be acquainted with corresponding…

Manufacturing Operations and Controls In Pharma Industry
Manufacturing Operations and Controls In Pharma Industry All manufacturing operations shall be carried out under the supervision of technical staff approved by the Licensing Authority. Each critical step in the process relating to the selection, weighing, and measuring of raw material addition during various stages shall be performed by trained personnel under the direct personal…

Disposal of waste
Disposal of waste Disposal of waste means eliminating, scraping, recycling, or destroying unwanted materials known as waste that is generated from the manufacturing, packing, and warehousing of industrial pharmaceutical products. Following the correct methods for waste disposal will ensure lesser pollution and hazards to the environment. All the pharmaceutical waste shall be collected initially in…

Handling of external laboratory activities
Handling of external laboratory activities To lay down a procedure for qualifying, auditing, sending samples with reference to testing procedures, and obtaining the results for the same. This SOP is applicable to all the external laboratories to which the samples are sent for testing due to the non-availability of in-house facilities/resources. PROCEDURE The external laboratory…

Procedure of laundry
Procedure of laundry To lay down the procedure for describing the steps to be followed for laundry the uniform. This Procedure is applicable to laundry. PROCEDURE Uniforms should be transferred from the production area in special closed plastic bags separately for each section like Packing, core area, and Warehouse. Clothes should be washed in accordance…

EXCEL SHEET VALIDATION
EXCEL SHEET VALIDATION The objective of this procedure is to establish the guideline for the validation of Microsoft Excel spreadsheets use for calculating the product quality parameters. This procedure covers the validation procedure for validation of Microsoft Excel spreadsheets use for calculating the product quality parameters in QA. Procedure Spreadsheets used for simple calculations (e.g:…

CALIBRATION OF DIES REQUIRED FOR BLEND SAMPLING
CALIBRATION OF DIES REQUIRED FOR BLEND SAMPLING The objective of this procedure is to establish the guideline for the calibration of dies required for blend sampling. This SOP is applicable to dies required for a sampling of the blend for validation batches manufactured in the Pharma Industry. Procedure Take a die to be calibrated for…

HANDLING COUNTERFEIT/FALSIFIED MATERIALS
HANDLING COUNTERFEIT/FALSIFIED MATERIALS To lay down a standard procedure that describes handling counterfeit/falsified materials. Applicable for the procedure of handling counterfeit/falsified materials. PROCEDURE The received material should undergo incoming inspection. If the incoming inspection results of the materials are unsatisfactory, the QA department initiates the investigation of the case. The Authorized person prepares a report…

Technology Transfer
Technology Transfer To lay down a procedure for technology transfer of new products to be taken. The scope of this procedure is to all the new Products for which Technology has been provided by an outside party PROCEDURE Whenever any NEW PRODUCT is to be taken at Pharmaceutical Industry & the technology has been provided…

Shipping Validation
Shipping Validation Objective The objective of this study is to establish a procedure to record temperature data to ensure that transportation conditions have not adversely affected a product until it reaches the Central Warehouse when transported as per regular Shipment practice. Scope The protocol shall be applicable at the formulation Plant. The Shipping (Transportation) validation…

COATING PANS
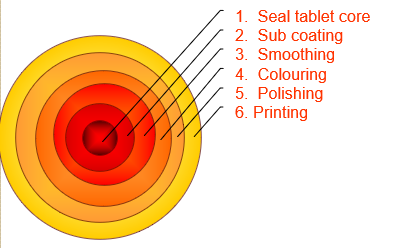
COATING PANS CONTENTS: COATING PROCESSES TYPES OF COATING PANS CONVENTIONAL COATING PANS PERFORATED COATING PANS VALIDATION OF COATING PANS IQ OQ PQ REFERENCES Main coating processes Film coating Sugar coating Sugar coating Traditionally sugar coatings formed the bulk of coated tablets but today film coatings are the more modern technology in tablet coating. Description of…