Your cart is currently empty!
Author: Alvina
Physical and chemical quality Control test of potable/raw water
Physical and chemical quality Control test of potable/raw water The procedure of potable/raw water quality control test. Raw water analysis report: Attachment-I Raw water analysis protocol: Attachment-II PROCEDURE Use a 500 ml vessel with a stopper for sampling. On the vessel write the name of sample, place and date of sampling. Allow water to…
Procedure for sampling of material for microbiological analysis
Procedure for a sampling of material for microbiological analysis To lay down the procedure for the sampling material for microbiological analysis. This SOP is applicable for the sampling of material for microbiological analysis. PROCEDURE: Sampling procedure Sample the material as per the sampling plan. The sampling device should be sterilized by autoclaving or thoroughly wiped…
Testing and reporting of in-process unpacked products
Testing and reporting of in-process unpacked products OBJECTIVE To provide the procedure of testing and reporting of in-process unpacked products. SCOPE It is applicable to the procedure of testing and reporting of in-process unpacked products. RESPONSIBILITY Quality Control Analyst shall be responsible for following the procedure mentioned in this SOP. ACCOUNTABILITY QC Head and QA…
Structural working in the physical-and-chemical laboratory
Structural working in the physical-and-chemical laboratory OBJECTIVE To lay down a procedure for the structural working in the physical-and-chemical laboratory. SCOPE This SOP is applicable for organization of work in the physical-and-chemical laboratory. RESPONSIBILITY Quality Control Analyst shall be responsible to follow the procedure mentioned in this SOP. ACCOUNTABILITY Quality Control and Head QA Head…
Procedures for organizations and undertake microbiological tests
Define procedures for organizations and undertake microbiological tests and the checking the applicable methods. The acknowledgment of the validity of the methods of the work. PROCEDURE: In conducting research in the microbiology laboratory staff must adhere to the established regime and anti-epidemic work rules. Persons permitted to work in the microbiology laboratory only those who…
Operation & Cleaning of Lift in Warehouse
Operation & Cleaning of Lift in Warehouse Objective: To lay down a procedure of operation & cleaning of lift in the warehouse. Scope: This procedure is applicable into warehouse . Responsibility: Warehouse supervisory staff shall be responsible for operation and cleaning of Lift. Accountability: Head Warehouse shall be accountable for implementation and compliance of this…
Procedures and Specifications for Media Fills
Procedures and Specifications for Media Fills The procedures and specifications used for media fills shall be described Summaries of results for validation using the same container- closure system shall be described Filling process that is to be used for the product should be described. The microbiological testing method(s) used should be described. Any procedural differences…

Cleaning Of Building Premises
Cleaning Of Building Premises OBJECTIVE: To lay down a standard procedure for the cleaning of building premises. SCOPE: This SOP is applicable for cleaning of entire Premises (Administration office, Utility, Security, Panel room, Change rooms, Toilets, Stairs, and Corridors, and outside areas including roads) . RESPONSIBILITY: Supervisor/Above – House Keeping and Human Resource & Administration…

WHO INSPECTION & REQUIRED DOCUMENTS
WHO INSPECTION & REQUIRED DOCUMENTS Objectives of a manufacturing site inspection The overall intent of a manufacturing site inspection is to assess the safety, performance, and quality of the Manufacturing site. The specific objectives are to assess compliance of the manufacturer’s quality management system (QMS) and manufacturing practices with international standards in order to: Determine…
AUTOCLAVE – MAINTENANCE AND VALIDATION
AUTOCLAVE – MAINTENANCE AND VALIDATION Qualification and Validation With such importance placed on the autoclave how can the user be certain that it has achieved the required sterilization? As an autoclave operator involved in your manufacturing process – or as a laboratory operator – you are responsible for your manufacturing process or for the results…

Changes as per SUPAC Guideline Part -I (components or composition)
Changes as per SUPAC Guideline Part -I (components or composition) During the post-approval period, to change: 1) The components or composition 2) The site of manufacture 3) The scale-up/scale-down of manufacture. 4) The manufacturing (process and equipment) of an immediate release oral formulation. The guidance defines: 1) Levels of change 2) Recommended chemistry,manufacturing, and controls…

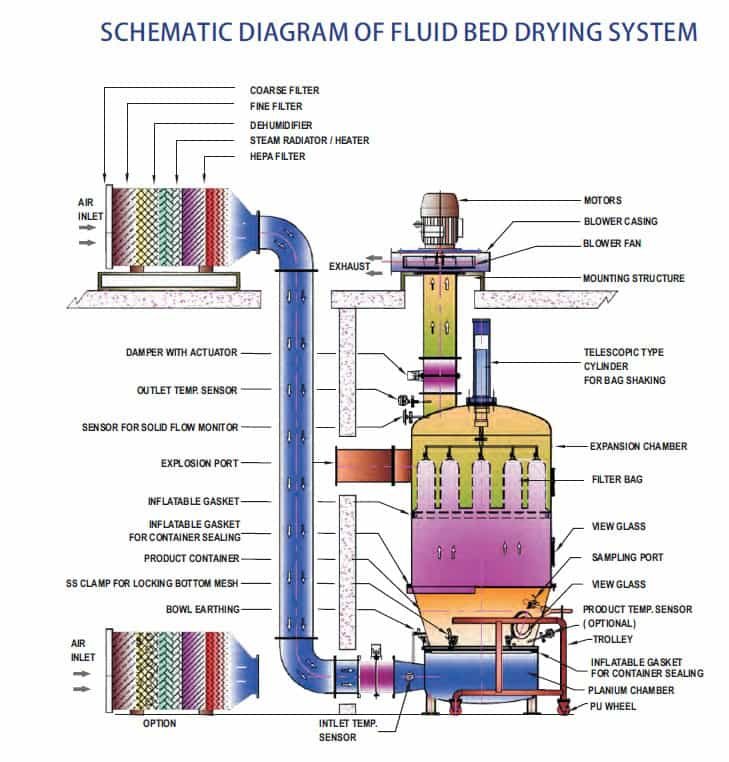
Operational Qualification of Fluid Bed Processor In Pharma
Operational Qualification of Fluid Bed Processor In Pharma Operational qualification is the process of demonstrating that Fluid Bed Processor will function according to its operational specification in the selected environment. All the operation of Fluid Bed Processor t is verified by performing the test functions,• A conclusion is drawn regarding the operation of Fluid Bed…

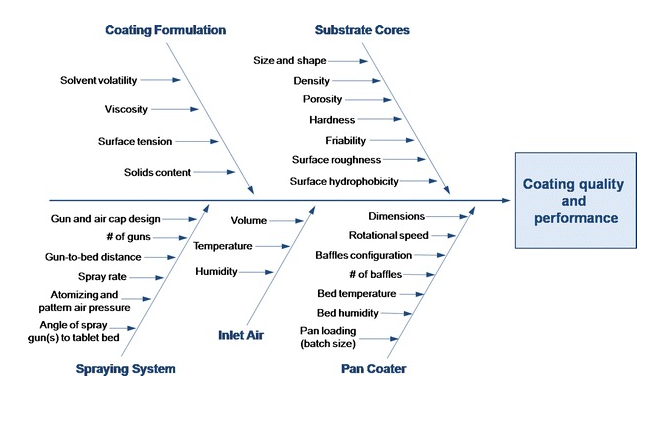
Film Coating By by design of experiments
Film Coating By by design of experiments To identify and screen the critical process parameters by design of experiments and optimizing it using the “Environmental Equivalency” (EE) factor. Film-coating of tablets has many variables and the factors like core tablet composition, coating equipment, process conditions and coating suspension, influence on quality and visual appearance of…

Quality Assurance Policies and QMS Procedure
Quality Assurance Policies and QMS Procedure Quality Assurance Policies Document and Data Control Policies Cleaning and Sanitization Policies Personnel Hygiene Policies Environmental Control Policies Pest Control Policies Training Policies Risk assessment policies Validation Policies Vendor Policies Quality Management System Site Master File Validation Master Plan Quality Manual, SOPs STPs Specifications Quality Management Procedures Change Control…

SOP for Responsibility of Compounding Personnel in sterile preparation
SOP for Responsibility of Compounding Personnel in sterile preparation Compounding personnel is responsible for ensuring that Compounding sterile preparations (CSPs) are accurately identified, measured, diluted, and mixed and are correctly purified, sterilized, packaged, sealed, labeled, stored, dispensed, and distributed. These performance responsibilities include maintaining appropriate cleanliness conditions and providing labeling and supplementary instructions for the proper…

Documentation for Manufacture of Pharma Sterile Products
Documentation for Manufacture of Pharma Sterile Products The Documents relating to the manufacture of sterile products shall be as follows but not limited to these documents only: – (1) Serial number of the Batch Manufacturing Record. (2) Name of the product. (3) Reference to Master Formula Record. (4) Batch / Lot number. (5) Batch /…

SOP for Out of Specification (OOS)
SOP for Out of Specification (OOS) SOP for the procedure for handling, investigating, and reporting test results, which do not meet the specification. This SOP is applicable to all out of specification results during the analysis of raw materials, packaging material, finished products, stability study samples, and any unusual results observed for test parameters This…
Registration of the samples received by the QC physical-and-chemical and microbiology laboratories for testing
Registration of the samples received by the QC physical-and-chemical and microbiology laboratories for testing OBJECTIVE To provide the registration of samples received by the QC physical-and-chemical and microbiology laboratories for testing. SCOPE This SOP is applicable to the registration of samples received by the QC physical-and-chemical and microbiology laboratories for testing. RESPONSIBILITY Quality Control Analyst…
Physical-and-chemical test analysis analytical reports
Physical-and-chemical test analysis analytical reports OBJECTIVE To provide the procedure analytical reports/calculation sheet. SCOPE This SOP is applicable to the the procedure analytical reports/calculation sheet. RESPONSIBILITY Quality Control Analyst shall be responsible for following the procedure mentioned in this SOP. ACCOUNTABILITY QC Head and QA Head shall be accountable for compliance of this SOP. ATTACHMENTS …
ACCELERATED STABILITY AND SHELF-LIFE CALCULATION
ACCELERATED STABILITY AND SHELF-LIFE CALCULATION Objective: To lay down a method for accelerated stability and to find out the shelf life of the product. SCOPE: To ensure that the procedure method for accelerated stability and to find out the shelf life to the product in pharmaceutical Industries. RESPONSIBILITY: QA and QC personnel shall be responsible…