Your cart is currently empty!
Tag: Iowa
AUTOCLAVE – MAINTENANCE AND VALIDATION
AUTOCLAVE – MAINTENANCE AND VALIDATION Qualification and Validation With such importance placed on the autoclave how can the user be certain that it has achieved the required sterilization? As an autoclave operator involved in your manufacturing process – or as a laboratory operator – you are responsible for your manufacturing process or for the results…

Changes as per SUPAC Guideline Part -I (components or composition)
Changes as per SUPAC Guideline Part -I (components or composition) During the post-approval period, to change: 1) The components or composition 2) The site of manufacture 3) The scale-up/scale-down of manufacture. 4) The manufacturing (process and equipment) of an immediate release oral formulation. The guidance defines: 1) Levels of change 2) Recommended chemistry,manufacturing, and controls…

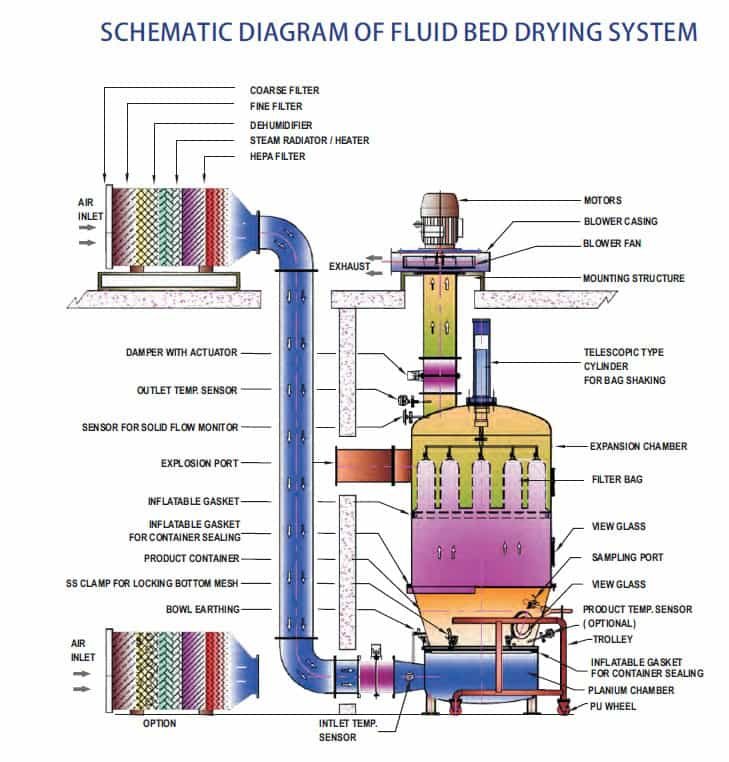
Operational Qualification of Fluid Bed Processor In Pharma
Operational Qualification of Fluid Bed Processor In Pharma Operational qualification is the process of demonstrating that Fluid Bed Processor will function according to its operational specification in the selected environment. All the operation of Fluid Bed Processor t is verified by performing the test functions,• A conclusion is drawn regarding the operation of Fluid Bed…

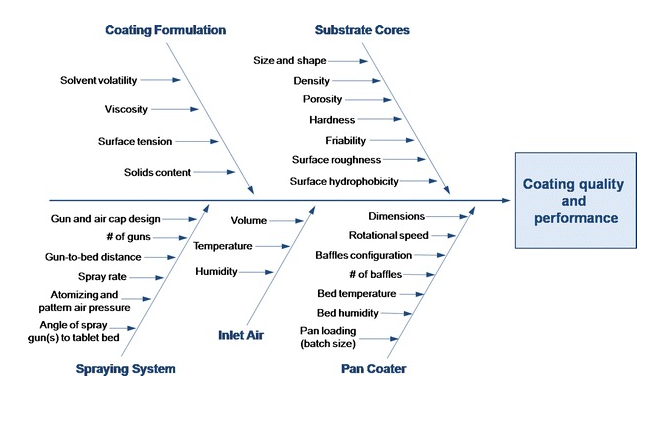
Film Coating By by design of experiments
Film Coating By by design of experiments To identify and screen the critical process parameters by design of experiments and optimizing it using the “Environmental Equivalency” (EE) factor. Film-coating of tablets has many variables and the factors like core tablet composition, coating equipment, process conditions and coating suspension, influence on quality and visual appearance of…

SOP for Responsibility of Compounding Personnel in sterile preparation
SOP for Responsibility of Compounding Personnel in sterile preparation Compounding personnel is responsible for ensuring that Compounding sterile preparations (CSPs) are accurately identified, measured, diluted, and mixed and are correctly purified, sterilized, packaged, sealed, labeled, stored, dispensed, and distributed. These performance responsibilities include maintaining appropriate cleanliness conditions and providing labeling and supplementary instructions for the proper…

Documentation for Manufacture of Pharma Sterile Products
Documentation for Manufacture of Pharma Sterile Products The Documents relating to the manufacture of sterile products shall be as follows but not limited to these documents only: – (1) Serial number of the Batch Manufacturing Record. (2) Name of the product. (3) Reference to Master Formula Record. (4) Batch / Lot number. (5) Batch /…

SOP for Out of Specification (OOS)
SOP for Out of Specification (OOS) SOP for the procedure for handling, investigating, and reporting test results, which do not meet the specification. This SOP is applicable to all out of specification results during the analysis of raw materials, packaging material, finished products, stability study samples, and any unusual results observed for test parameters This…